- Product Description and Classification
- Mandatory Requirements
- Affirmation of Compliance Codes for Foods
- What are the Requirements for the Niche Markets?
- Which Quality Support Organizations in Lebanon Can Help Me?
Product Description and Classification
Pickles and pickled products consist of a variety of fruits and vegetables that have been conserved through a mixture of acidic solution, salt, and spices.There are six basic types of ingredients used in the process: the main food that will be pickled, acids or brine, colorants, flavorings, preservatives, and stabilizers that constitute the liquid in which the pickled product is sold.The pickling process is based on two main components: the lactic acid fermentation as well as the vegetables that may be salted or not, which would in turn result in different end products, tastes, and textures.Hygiene practices are crucial in the pickling process, as the fermentation process does not require the heating of vegetables and fruits.The lactic acid bacteria brew sugar during the fermentation process into lactic acid, thus avoiding the formation of toxic bacteria and fungus in the pickling process.In addition, salt is a key component in the process as it affects the level and form of fermentation, and therefore it is recommended that the bacteria grow in a low concentration of salt.In this context, 2 % to 5 % of salt will yield pickles with high levels of acidity, whereas higher levels of salt(up to 16 % ) will result in salt - stock pickles or pickles with high salt concentration.Moreover, sugar is among the inputs that are sometimes added to sweeten the pickles or to increase the degree of fermentation.
It is also important to mention that the temperature and the level of pH should be monitored in order to avoid the development of unwanted bacteria.Combined Nomenclature Number Product 200110 Cucumbers and gherkins, prepared or preserved by vinegar or acetic acid 200190 Vegetables, fruits, nuts and other edible parts of plants, prepared or preserved by vinegar or acetic acid(excluding cucumbers and gherkins)
| Combined Nomenclature Number | Product |
|---|---|
| 200110 | Cucumbers and gherkins, prepared or preserved by vinegar or acetic acid |
| 200190 | Vegetables, fruits, nuts and other edible parts of plants, prepared or preserved by vinegar or acetic acid (excluding cucumbers and gherkins) |
Mandatory Requirements
All FDA-regulated products imported into the United States are required to meet the same laws and regulations as domestic goods. Moreover, imported foods must be pure, wholesome, safe to eat, and produced under sanitary conditions; drugs and devices must be safe and effective; cosmetics must be safe for their intended use and properly labeled; radiation-emitting devices must meet established standards; tobacco products must meet U.S. requirements; and all the products must contain informative and truthful labeling in English.FDA-regulated products are subject to inspection when offered for import into the United States. Products may be refused entry if they appear, from examination or otherwise, to violate FDA requirements. Some products are subject to certification and/or testing requirements due to a past history of violations; these products are identified in import alerts and in certain international cooperative arrangements.
All imported shipments of FDA-regulated products are reviewed by the FDA and must comply with the same standards as domestic products. In other words, the FDA determines whether products are admissible into U.S. commerce and may refuse entry of any product that violates or appears to violate any provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
Furthermore, the FDA receives many different types of entries (consumption, informal, warehouse, import for export, etc.). Most questions revolve around the difference between commercial and personal shipments.
- Commercial Shipments: Imported goods brought into U.S. commerce for sale or distribution. To find out more information about the different types of entries visit our Common Entry Types page.
- Personal Shipments: Imported goods brought into the U.S. for personal use.
During the entry review process , the imported products must be held and may not be distributed into the U.S. commerce until the FDA has determined their admissibility. FDA-regulated products are refused entry if they appear to be or have been found to be:
- adulterated — meaning the product is contaminated, not safe, unapproved, or does not otherwise meet applicable standards
- misbranded — meaning the labels contain false or misleading information, or the product is not registered and listed (if required)
- forbidden or restricted for sale
The Center for Food Safety and Applied Nutrition (CFSAN) is the FDA center responsible for overseeing the human food program. Visit the Food webpage for more information.
- Prior Notice Requirements for Importation
- Food Facility Registration
- Low Acid Canned Foods/Acidified Foods (LACF/AF)
- Food Safety Modernization Act (FSMA)
- Food Labeling
Prior Notice Requirements for Importation
The FDA must receive notification before the food is offered for import into the U.S. This measure — giving prior notice — is done in order to enable the FDA to effectively target inspections or examinations of the imported food at the U.S. ports of entry, and in order to determine whether the imported food shipment presents any threat or serious risk to public health, with the condition that it is based on credible information.
Imported foods must be pure, wholesome, safe to eat, produced under sanitary conditions, and contain informative and truthful labeling in English. The FDA does not certify, license, or otherwise approve individual food importers, products, labels, or shipments prior to importation.
At the time of importation, the FDA will verify compliance with the following requirements:
Federal Regulations require commercial processors of shelf-stable acidified foods and low-acid canned foods in a hermetically sealed container that are to be sold in the U.S. to register each establishment and file scheduled processes with the Food and Drug Administration for each product, product style, container size, and type and processing method (21 CFR 108). This website contains instructions for establishment registration and process filing along with other information useful to manufacturers of these types of products.
A low-acid canned food (LACF) is any food (other than alcoholic beverages) with a finished equilibrium pH greater than 4.6 and a water activity greater than 0.85, excluding tomatoes and tomato products which have a finished equilibrium pH less than 4.7.
An acidified food (AF) is a low-acid food to which acid(s) or acid food(s) are added and which has a finished equilibrium pH of 4.6 or below and a water activity (aw) greater than 0.85.
Reference: https://www.fda.gov/food/guidance-documents-regulatory-information-topic/guidance-industry-low-acid-foods-packaged-hermetically-sealed-containers-lacf-regulation-and-fda
Imported foods must be pure, wholesome, safe to eat, produced under sanitary conditions, and contain informative and truthful labeling in English. The FDA does not certify, license, or otherwise approve individual food importers, products, labels, or shipments prior to importation.
At the time of importation, the FDA will verify compliance with the following requirements:
Federal Regulations require commercial processors of shelf-stable acidified foods and low-acid canned foods in a hermetically sealed container that are to be sold in the U.S. to register each establishment and file scheduled processes with the Food and Drug Administration for each product, product style, container size, and type and processing method (21 CFR 108). This website contains instructions for establishment registration and process filing along with other information useful to manufacturers of these types of products.
A low-acid canned food (LACF) is any food (other than alcoholic beverages) with a finished equilibrium pH greater than 4.6 and a water activity greater than 0.85, excluding tomatoes and tomato products which have a finished equilibrium pH less than 4.7.
An acidified food (AF) is a low-acid food to which acid(s) or acid food(s) are added and which has a finished equilibrium pH of 4.6 or below and a water activity (aw) greater than 0.85.
Reference: https://www.fda.gov/food/guidance-documents-regulatory-information-topic/guidance-industry-low-acid-foods-packaged-hermetically-sealed-containers-lacf-regulation-and-fda
Food Facility Registration
Most facilities that manufacture, process, pack, receive, or hold food must register with the FDA every two years. This includes most foreign manufacturers and some importers.
Low Acid Canned Foods/Acidified Foods (LACF/AF)
Importers of low-acid and acidified foods who import low-acid canned food or acidified food products into the U.S. are not required to register and file processes; however, they must ensure that the processing firms, which they represent, comply with all the registration and process filing requirements.
The FDA verifies that commercial processors engaged in the manufacturing, processing, or packing of acidified foods (AF) or low-acid canned foods (LACF) are registered and have a process on file for each product they import.
Reference: https://www.fda.gov/food/acidified-low-acid-canned-foods-lacf-registration/establishment-registration-and-process-filing-acidified-and-low-acid-canned-foods-lacf-electronic
The FDA verifies that commercial processors engaged in the manufacturing, processing, or packing of acidified foods (AF) or low-acid canned foods (LACF) are registered and have a process on file for each product they import.
Reference: https://www.fda.gov/food/acidified-low-acid-canned-foods-lacf-registration/establishment-registration-and-process-filing-acidified-and-low-acid-canned-foods-lacf-electronic
Food Safety Modernization Act (FSMA)
It gives the FDA new tools and authorities to ensure imported foods (for humans and animals) meet the same safety standards as foods produced in the U.S.
importers have an explicit responsibility of verifying that their foreign suppliers have adequate preventive controls in place to ensure that the food they produce is safe.
Reference: https://www.federalregister.gov/articles/2015/11/27/2015-28158/foreign-supplier-verification-programs-for-importers-of-food-for-humans-and-animals
importers have an explicit responsibility of verifying that their foreign suppliers have adequate preventive controls in place to ensure that the food they produce is safe.
Reference: https://www.federalregister.gov/articles/2015/11/27/2015-28158/foreign-supplier-verification-programs-for-importers-of-food-for-humans-and-animals
Food Labeling
Food labeling is required for most prepared foods, such as bread, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling is also required for raw produce (fruits and vegetables).
i. Labels must bear a Statement of Identity
Every food label must bear a statement of identity, also known as the name of the product. The common or usual name of the food should be used if it has one. The statement of identity must be placed on the principal display panel (PDP), as well as any alternate PDP. The statement of identity must be in bold type and should be one of the most prominent features on the PDP. Some foods have standards of identity. In other words, standards of identity define criteria that a product must meet in order to identify by a certain name. For example, in order to identify as “blue cheese,” a product must, among other things, have a maximum moisture content of 46 percent by weight, must contain the mold Penicillium roquefortii, and must be at least 60 days old. Moreover, products with standards of identity include, but are not limited to, milk and creams; types of cheeses; ice cream and other frozen desserts; bread, rolls, and buns; and cereal flours.
ii. Labels must bear the required Nutrition Facts Chart
The FDA requires food labels to bear a Nutrition Facts Chart. The Nutrition Facts Charts contain information such as a serving size, the number of calories the product contains, as well as the amount of fat, sodium, protein, and other ingredients in the product. The FDA has a specific format that Nutrition Facts Charts must follow; this includes everything from the order of the content to the font sizes. The Nutrition Facts Charts should be placed with the ingredient list and the name and address of the manufacturer, packer, or distributor on the PDP or the information panel of the food label. If there is insufficient space, the Nutrition Facts Charts may be placed on an alternate panel that is easily viewable to the consumer. In February 2014, FDA proposed the following changes to the Nutrition Facts Chart:
iii. Labels must list each ingredient used in a food product
The FDA requires that every ingredient contained within a food or beverage be disclosed on the label in descending order of predominance by weight. Ingredients should always be listed by their common or usual name unless a regulation provides a different term. For example, “sugar” is often used in place of “sucrose.” It’s important to always include major food allergens in the ingredient list, no matter how small the amount. Unlisted allergens are the leading cause of FDA-requested recalls. Major food allergens include milk, eggs, fish, shellfish, tree nuts, wheat, peanuts, and soybeans.
iv. Labels must be printed in English
The FDA requires that all mandatory words, statements, and other information on labels appear in English. A label may include other languages along with English; however, if a foreign language is used anywhere on the label, all of the required information on the label must appear in the foreign language as well.
v. Labels cannot bear inappropriate claims
The FDA has strict regulations for claims made on food labels. Some types of claims may be used as long as a product meets certain criteria, while other claims require the FDA’s evaluation. There are three main types of claims that can be made on food labels:
i. Labels must bear a Statement of Identity
Every food label must bear a statement of identity, also known as the name of the product. The common or usual name of the food should be used if it has one. The statement of identity must be placed on the principal display panel (PDP), as well as any alternate PDP. The statement of identity must be in bold type and should be one of the most prominent features on the PDP. Some foods have standards of identity. In other words, standards of identity define criteria that a product must meet in order to identify by a certain name. For example, in order to identify as “blue cheese,” a product must, among other things, have a maximum moisture content of 46 percent by weight, must contain the mold Penicillium roquefortii, and must be at least 60 days old. Moreover, products with standards of identity include, but are not limited to, milk and creams; types of cheeses; ice cream and other frozen desserts; bread, rolls, and buns; and cereal flours.
ii. Labels must bear the required Nutrition Facts Chart
The FDA requires food labels to bear a Nutrition Facts Chart. The Nutrition Facts Charts contain information such as a serving size, the number of calories the product contains, as well as the amount of fat, sodium, protein, and other ingredients in the product. The FDA has a specific format that Nutrition Facts Charts must follow; this includes everything from the order of the content to the font sizes. The Nutrition Facts Charts should be placed with the ingredient list and the name and address of the manufacturer, packer, or distributor on the PDP or the information panel of the food label. If there is insufficient space, the Nutrition Facts Charts may be placed on an alternate panel that is easily viewable to the consumer. In February 2014, FDA proposed the following changes to the Nutrition Facts Chart:
- Include “added sugars”
- Update the daily values for sodium, dietary fiber, and Vitamin D
- Include the amount of potassium and Vitamin D
- Remove “Calories from Fat”
- Update serving sizes to more realistically reflect American consumption
iii. Labels must list each ingredient used in a food product
The FDA requires that every ingredient contained within a food or beverage be disclosed on the label in descending order of predominance by weight. Ingredients should always be listed by their common or usual name unless a regulation provides a different term. For example, “sugar” is often used in place of “sucrose.” It’s important to always include major food allergens in the ingredient list, no matter how small the amount. Unlisted allergens are the leading cause of FDA-requested recalls. Major food allergens include milk, eggs, fish, shellfish, tree nuts, wheat, peanuts, and soybeans.
iv. Labels must be printed in English
The FDA requires that all mandatory words, statements, and other information on labels appear in English. A label may include other languages along with English; however, if a foreign language is used anywhere on the label, all of the required information on the label must appear in the foreign language as well.
v. Labels cannot bear inappropriate claims
The FDA has strict regulations for claims made on food labels. Some types of claims may be used as long as a product meets certain criteria, while other claims require the FDA’s evaluation. There are three main types of claims that can be made on food labels:
- Health Claims — characterize the relationship of any substance to a disease or health-related condition and are limited to claims about disease risk reduction. Health claims must be supported by scientific evidence and must be reviewed by the FDA before they are used.
- Nutrient Content Claims — characterize the level of a nutrient in a food (e.g., “low fat”, “good source of fiber”, etc.).
- Structure/Function Claims — describe the role of a nutrient or dietary ingredient intended to affect the normal structure of function in humans. It’s important to be careful about the wording of claims. In other words, claims that state or imply that a product can diagnose, cure, mitigate, or treat a disease may cause a food product to be regulated as a drug. In this case, the product would be considered an unapproved new drug and therefore adulterated if distributed into U.S. commerce.
Affirmation of Compliance Codes for Foods
Affirmation of Compliance codes (A of C) are three-letter codes that can be provided at the time of import to facilitate the FDA review. The FDA uses A of C codes to assist in verifying that your product meets the appropriate requirements. Providing the correct A of C codes reduces the likelihood that your shipment will be held for further FDA entry review during the FDA’s import screening process. Qualifiers provide product and manufacturer-specific information. In turn, the FDA uses this information to verify that the product is in compliance. Some affirmations require a product manufacturer’s registration number, (unique to the facility) or a product’s approval number (specific to the product). Reference: https://www.fda.gov/media/97480/download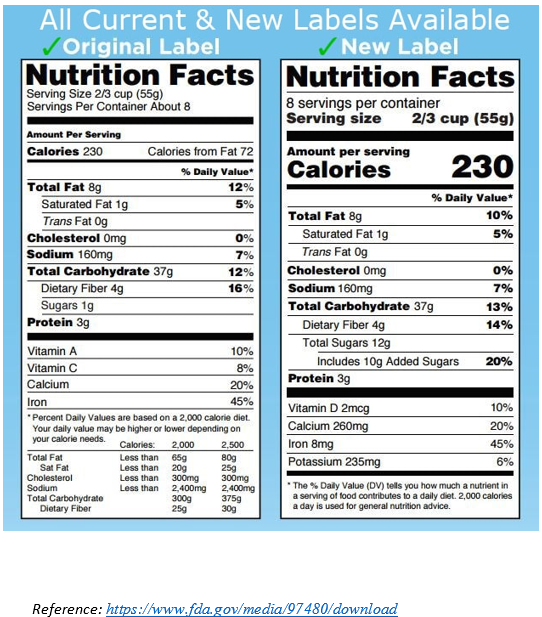
What are the Requirements for the Niche Markets?
Organic Certification
In the US, the FDA does not regulate the use of the term “organic” on food labels. The USDA requirements for products that are labeled with the term "organic" are separate from the laws that the FDA enforces. Moreover, food products that are ordinarily under the FDA's jurisdiction and that are labeled with organic claims must comply with both the USDA NOP regulations for the organic claim and the FDA regulations for food labeling and safety.
The National Organic Program (NOP), part of USDA’s Agricultural Marketing Service (AMS), establishes international organic import and export policies to facilitate trade and to expand market opportunities for certified organic farms and businesses around the world.
Imported organic products must be certified to one of the following standards to be sold in the U.S.:
Certifiers are responsible for making sure that the USDA organic products meet all organic standards. There are five basic steps to organic certification:
The National Organic Program (NOP), part of USDA’s Agricultural Marketing Service (AMS), establishes international organic import and export policies to facilitate trade and to expand market opportunities for certified organic farms and businesses around the world.
Imported organic products must be certified to one of the following standards to be sold in the U.S.:
- The USDA organic regulations — USDA authorizes organizations around the world to certify farms and businesses to the USDA organic regulations.
- An authorized international standard — the U.S. has established trade partnerships with international countries.
Certifiers are responsible for making sure that the USDA organic products meet all organic standards. There are five basic steps to organic certification:
- 1. The farm or business adopts organic practices, selects a USDA-accredited certifying agent, and submits an application and fees to the certifying agent.
- 2. The certifying agent reviews the application to verify that the practices comply with the USDA organic regulations.
- 3. An inspector conducts an on-site inspection of the applicant’s operation.
- 4. The certifying agent reviews the application and the inspector’s report to determine if the applicant complies with the USDA organic regulations.
- 5. The certifying agent issues an organic certificate.
Tips
- People who sell or label a product "organic" when they know it does not meet the USDA standards can be assessed a financial penalty with fines of several thousands of dollars for each violation
- In order to certify your product, you’ll need to access the list of certifier agents in the US For organic certifier agent check
- For organic rules and regulations check https://www.ams.usda.gov/rules-regulations/organic
- The USDA currently has organic equivalence with the following governments: Canada, European Union, Japan, Korea, Switzerland, Taiwan, and United Kingdom
Gluten-free Certification
The “gluten-free” definition is designed to protect individuals with celiac disease — a hereditary and chronic inflammatory disorder of the small intestine — who are advised to avoid all sources of gluten in their diet to protect against adverse health effects associated with the disease.
The U.S. Food and Drug Administration today released a final rule to establish compliance requirements for fermented and hydrolyzed foods, or foods that contain fermented or hydrolyzed ingredients, that bear the “gluten-free” claim. The final rule, titled “Gluten-Free Labeling of Fermented or Hydrolyzed Foods,” covers foods such as yogurt, sauerkraut, pickles, cheese, green olives, FDA-regulated beers and wines, and hydrolyzed plant proteins used to improve flavor or texture in processed foods such as soups, sauces, and seasonings.
Since gluten proteins in hydrolyzed and fermented foods are no longer intact and, currently, cannot be adequately detected and quantified through testing, the final rule states that FDA will determine compliance based on records kept by the manufacturer to show that their foods are gluten-free before fermentation or hydrolysis. It also includes a discussion of compliance with distilled foods such as vinegar.
Currently, the FDA knows of no scientifically valid analytical method effective in detecting and quantifying with precision the gluten protein content in fermented or hydrolyzed foods in terms of equivalent amounts of intact gluten proteins.
The U.S. Food and Drug Administration today released a final rule to establish compliance requirements for fermented and hydrolyzed foods, or foods that contain fermented or hydrolyzed ingredients, that bear the “gluten-free” claim. The final rule, titled “Gluten-Free Labeling of Fermented or Hydrolyzed Foods,” covers foods such as yogurt, sauerkraut, pickles, cheese, green olives, FDA-regulated beers and wines, and hydrolyzed plant proteins used to improve flavor or texture in processed foods such as soups, sauces, and seasonings.
Since gluten proteins in hydrolyzed and fermented foods are no longer intact and, currently, cannot be adequately detected and quantified through testing, the final rule states that FDA will determine compliance based on records kept by the manufacturer to show that their foods are gluten-free before fermentation or hydrolysis. It also includes a discussion of compliance with distilled foods such as vinegar.
Currently, the FDA knows of no scientifically valid analytical method effective in detecting and quantifying with precision the gluten protein content in fermented or hydrolyzed foods in terms of equivalent amounts of intact gluten proteins.
- - Thus, we plan to evaluate compliance of such fermented or hydrolyzed foods that bear a “gluten-free” claim based on records that are made and kept by the manufacturer of the food bearing the “gluten-free” claim and made available to us for inspection and copying. The records need to provide adequate assurance that the food or ingredients used in the food are “gluten-free” before fermentation or hydrolysis.
- - Once we identify that a scientifically valid method has been developed that can accurately detect and quantify gluten in fermented or hydrolyzed foods or ingredients, it would no longer be necessary for the manufacturer of foods bearing the “gluten-free” claim to make and keep these records. In addition, because there currently is no scientifically valid analytical method effective in detecting and quantifying the gluten protein content in fermented or hydrolyzed foods, the final rule requires the manufacturer of these kinds of foods bearing the “gluten-free” claim to document that it has adequately evaluated the potential for gluten cross-contact and, if identified, that the manufacturer has implemented measures to prevent the introduction of gluten into the food during the manufacturing process.
- - Likewise, the final rule requires manufacturers of foods that contain fermented or hydrolyzed ingredients and bear the “gluten-free” claim to make and keep records that demonstrate with adequate assurance that the fermented or hydrolyzed ingredients are “gluten-free” in compliance with the 2013 gluten-free food labeling final rule. Finally, this final rule states that the FDA will evaluate compliance with distilled foods by verifying the absence of protein using scientifically valid analytical methods that can reliably detect the presence of protein or protein fragments in the distilled food.

Ethnic Certification
Islamic dietary laws (Halal) propose specific restrictions on diets. If you want to focus on the Islamic ethnic niche markets, consider implementing Halal certification schemes.

Which Quality Support Organizations in Lebanon Can Help Me?
The Lebanese Agricultural Research Institute (LARI) is a governmental organization under the Minister of Agriculture’s supervision. The institute conducts applied and basic scientific research for the development and advancement of the agricultural sector in Lebanon. Extension services for farmers include management of soil fertility, water consumptive use, plant pest, and disease control, crop rotation, and animal disease treatment and prevention, among others.
The Lebanese Standards Institution (LIBNOR) is a public institution attached to the Ministry of Industry. It was established on July 23, 1962 by a law giving it solely the right to prepare, publish, and amend national standards, as well as to grant the Lebanese Conformity Mark NL. Lebanese standards are prepared by technical committees formed by LIBNOR, which include setting the dimensions, conventions, symbols, and the definition of products’ quality, as well as the methods of testing and analysis. They also include the codes of practice for professional and structural work.
The Industrial Research Institute (IRI) is registered as a Lebanese nonprofit institution. It provides, on an international scientific level, reliable services in testing and analysis and grants certificates of quality or conformity with standards and purchase specifications. It also provides specialized technological, management, and economic consulting services to existing industries and industrial development schemes.
The Chamber of Commerce, Industry, and Agriculture of Beirut and Mount Lebanon (CCIA-BML) is a non-profit private organization operating under Decree-Law 36/67. The Lebanese Chambers are the sole providers of consular services, including certification of origin and authentication of commercial documents. Also, the chambers conduct training, develop partnerships, and organize matchmaking events and exhibitions, among other services. The CCIA-BML operates the Lebanese Training Center, which provides managerial and technical training for Lebanese enterprises. In addition, the Chamber of Commerce, Industry, and Agriculture of Tripoli and North Lebanon provides quality control center laboratories, among other services.
The Lebanese Standards Institution (LIBNOR) is a public institution attached to the Ministry of Industry. It was established on July 23, 1962 by a law giving it solely the right to prepare, publish, and amend national standards, as well as to grant the Lebanese Conformity Mark NL. Lebanese standards are prepared by technical committees formed by LIBNOR, which include setting the dimensions, conventions, symbols, and the definition of products’ quality, as well as the methods of testing and analysis. They also include the codes of practice for professional and structural work.
The Industrial Research Institute (IRI) is registered as a Lebanese nonprofit institution. It provides, on an international scientific level, reliable services in testing and analysis and grants certificates of quality or conformity with standards and purchase specifications. It also provides specialized technological, management, and economic consulting services to existing industries and industrial development schemes.
The Chamber of Commerce, Industry, and Agriculture of Beirut and Mount Lebanon (CCIA-BML) is a non-profit private organization operating under Decree-Law 36/67. The Lebanese Chambers are the sole providers of consular services, including certification of origin and authentication of commercial documents. Also, the chambers conduct training, develop partnerships, and organize matchmaking events and exhibitions, among other services. The CCIA-BML operates the Lebanese Training Center, which provides managerial and technical training for Lebanese enterprises. In addition, the Chamber of Commerce, Industry, and Agriculture of Tripoli and North Lebanon provides quality control center laboratories, among other services.




